PRESSRELEASES
16 April, 2024
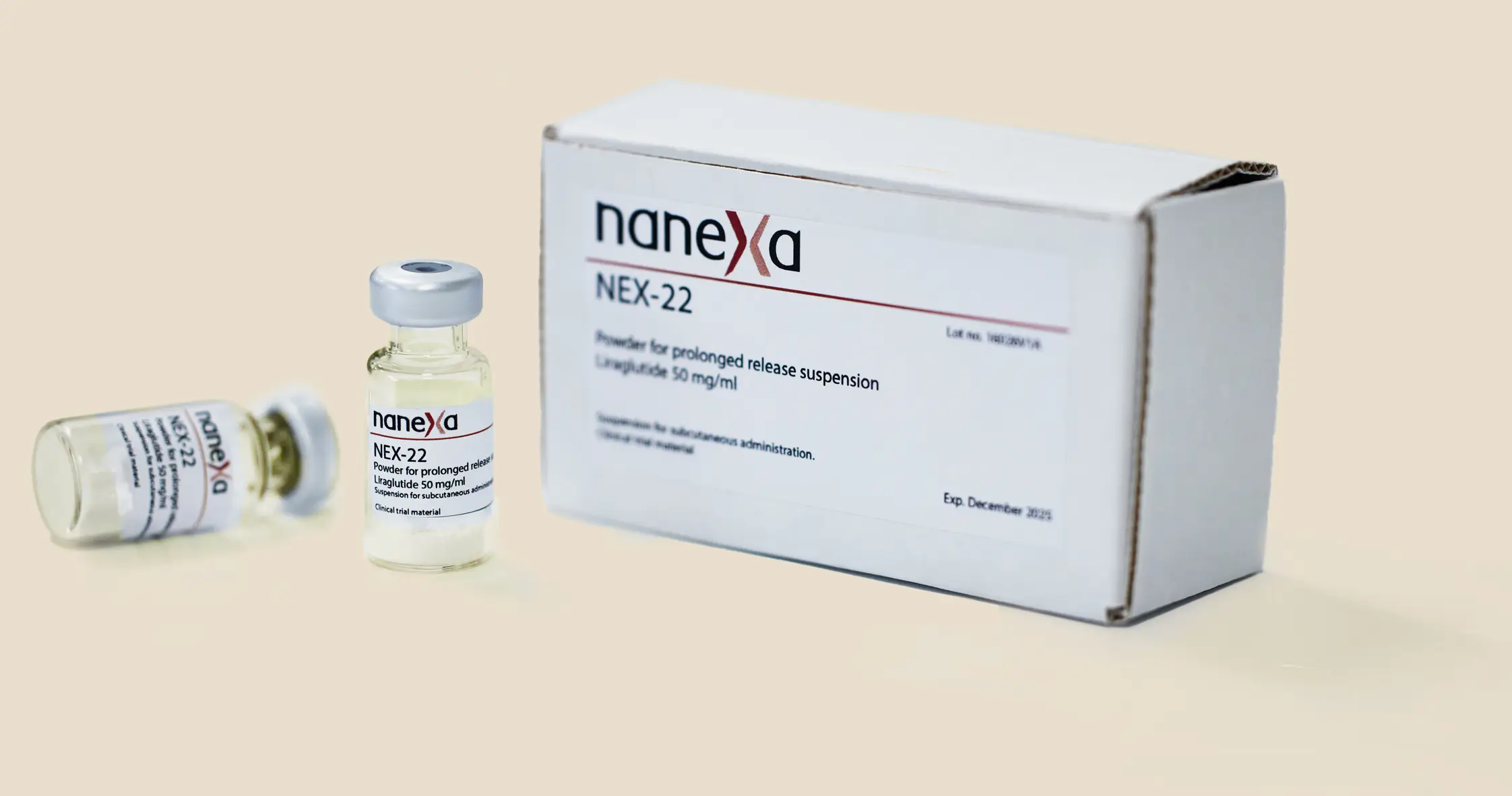
Approval of clinical trial application for NEX-22 study delayed
Nanexa AB today announces that in the ongoing review process of the clinical trial application for the phase I study with NEX-22, additional supplements have been requested from the German Medicines Agency, which in the new European regulatory process takes more time than expected.
READ MORE15 April, 2024
Notice of annual general meeting in Nanexa AB (publ)
The shareholders in Nanexa AB (publ), reg.no 556833–0285 (the ”Company”) are hereby given notice that the annual general meeting will be held on Wednesday 15 May 2024, at. 3:00 pm, at Uppsala Business Park, Rapsgatan 7, Uppsala, Sweden.
READ MORELATEST REPORTS
PRESENTATIONS
2024-03-08
ABG Sundal Collier – Fireside chat with CEO David Westberg
2023-10-24
Aktieportföljen Live, CEO David Westberg presents
2023-10-09