What is
How
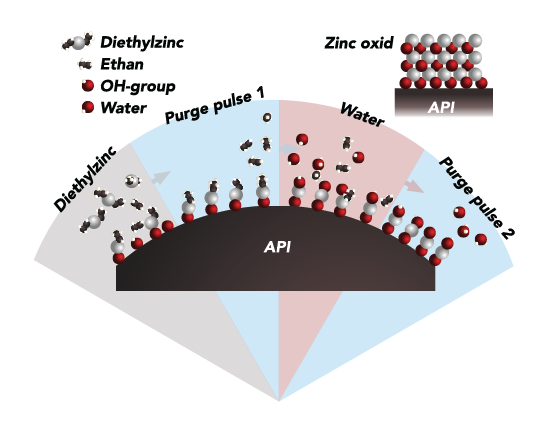
The formulation is a suspension containing coated drug particles with a controlled solubility of the coating. As the coating dissolves, the drug is released in a pre-defined manner over time.

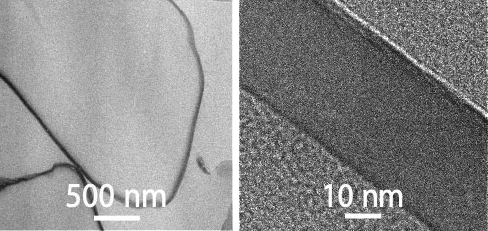
By customizing both the thickness and composition of this coating we can control the speed at which the API is released into the bloodstream over days, weeks or months.
Biologics
The high percentage of active substance in a PharmaShell®-formulation and the good syringeability enable small injection volumes with very thin needles. This is used in the NEX-22 project, one of Nanexa’s projects in clinical phase, where the goal is a month-long depot of the type 2 diabetes drug liraglutide.
In an in vivo mouse study, a water-soluble peptide was coated with PharmaShell® with a high drug load (>70% API). Whilst there was rapid clearance of the uncoated API, the initial release of the coated peptide showed a small initial burst release, Cmax/Css about 10×, then controlled release continuing for the full duration of the study: 8 days.
In a lysozyme protein model, enzyme activity was unaffected and there was a sustained in vitro release of coated protein.
ALD
Until 2022, Nanexa had a small-scale Atomic Layer Deposition manufacturing, fill and finish facility in Uppsala where it produced ALD coatings. The facility is GMP-certified by the Swedish Medical Products Agency for production of clinical trial material.
Nanexa is, together with its partner Applied Materials Inc, global leaders in ALD, scaling up its manufacturing capabilities in a new aseptic, two-line manufacturing plant for PharmaShell, which can handle production to up to kg batches, with filling and finishing onsite as well.
The completed pilot plant is the first GMP pharmaceutical manufacturing plant in the world with full Atomic Layer Deposition capabilities.