NEX-18
A new formulation of azacitidine, first-line treatment for MDS (myelodysplastic syndrome), a rare group of bone marrow failure disorders.
READ MORE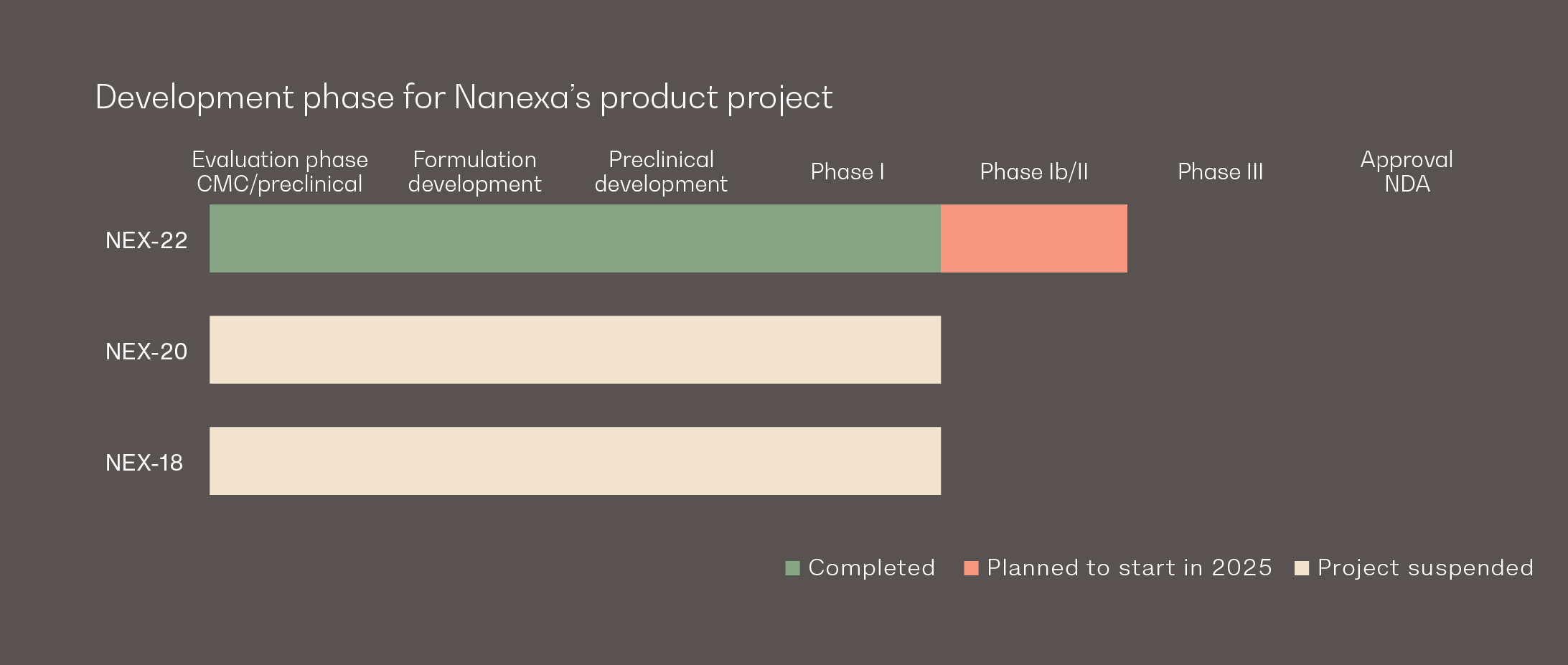
PARTNER COLLABORATIONS
In addition to its own product development projects, Nanexa also licenses the PharmaShell technology for other pharmaceutical companies to develop their own long-acting injectables.
The collaborations regarding licensing of the PharmaShell system normally starts with an evaluation of the technology through the coating of model substances or drug product candidates. Initially, a so-called Material Transfer and Feasibility Study Agreement (MTA) is signed, and Nanexa is paid for the services provided. If the partner company wants to move on with the ambition to develop a drug candidate, through additional preclinical and clinical studies, a license agreement is signed, covering technology access, production of clinical trial material and commercial rights at product launch. Such agreement typically includes technology access fee, milestone payments and royalties on sales of a final product.
Nanexa is continuously developing new and existing relations with potential partners Several companies evaluatesPharmaShell, for example Novo Nordisk and AstraZeneca.