Vad är
Hur
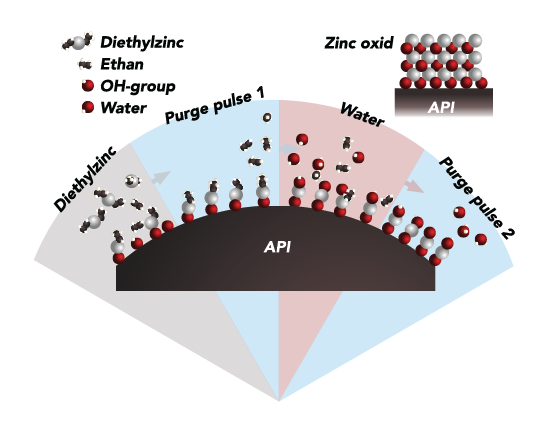
Formuleringen består av en suspension av ytbelagda läkemedelspartiklar vars skal har en kontrollerad löslighet. Läkemedlet frisätts successivt över tid, på ett fördefinierat sätt, i takt med att ytbeläggningen löses upp.

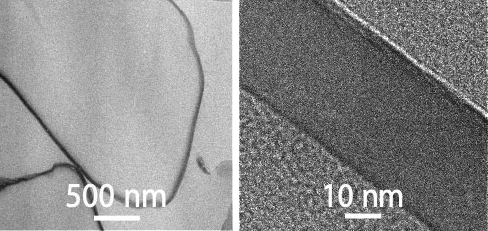
Genom att anpassa ytbeläggningens tjocklek och sammansättning kan vi styra hur snabbt den aktiva substansen frisätts i blodet, i perioder om dagar, veckor och månader.
Biologiska läkemedel
Den höga andelen aktiv substans i en PharmaShell®-formulering och den goda sprutbarheten möjliggör små injektionsvolymer med mycket tunna nålar. Detta utnyttjas i NEX-22 projektet, ett av Nanexas projekt i klinisk fas, där målet är en månadslång depå av typ 2-diabetesläkemedlet liraglutid.
Exempel: I en in-vivo studie i mus studerades frisättningen av en PharmaShell-belagd peptid där andelen peptid var över 70% efter beläggning med PharmaShell. Studien som varade i 8 dagar visade att vi hade en kontrollerad initial frisättning och att frisättningen fortsatte under hela studietiden.
I en lysozym-protein-modell var enzymaktiviteten oförändrad och det skedde en kontinuerlig in vitro-frisättning av de ytbelagda proteinerna.
ALD
Fram till 2022 bedrev Nanexa en småskalig anläggning för ALD-baserad tillverkning, fyllning och paketering i Uppsala, där bolaget framställde ALD-ytskikten. Läkemedelsverket har GMP-certifierat anläggningen för tillverkning av kliniskt prövningsmaterial.
Tillsammans med samarbetspartnern Applied Materials Inc, en av världens främsta ALD-aktörer, skalar Nanexa nu upp sina tillverkningsresurser i en ny, aseptisk tillverkningsanläggning med två produktionslinjer för PharmaShell. Här kan produktionsvolymer om upp till kilo-satser hanteras och även fyllning och paketering görs på plats.
Den färdigställda pilotanläggningen blir världens första GMP-certifierade anläggning för läkemedelsproduktion utrustad med kompletta Atomic Layer Deposition-resurser.